
Essential Organic Chemistry (3rd Edition)
3rd Edition
ISBN: 9780321937711
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17.1, Problem 1P
- a. Explain why, when the imidazole ring of histidine is protonated, the double-bonded nitrogen is the nitrogen that accepts the proton. (Hint: Localized electrons are more apt to be protonated than delocalized electrons.)
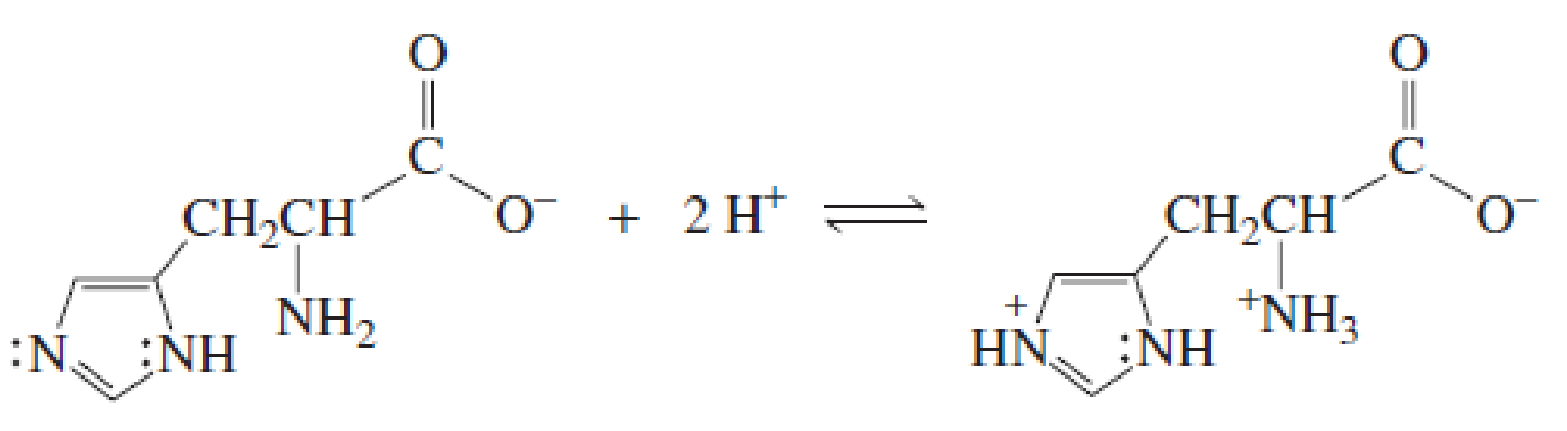
- b. Explain why, when the guanidino group of arginine is protonated, the double-bonded nitrogen is the nitrogen that accepts the proton.
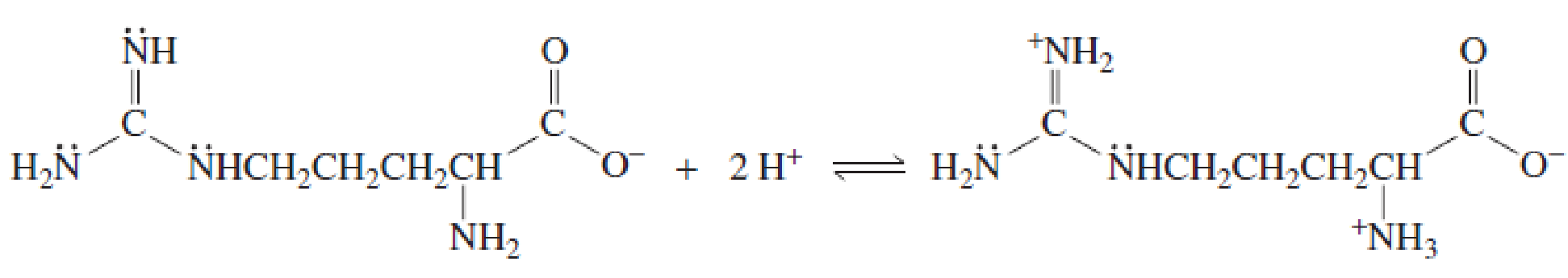
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
10. Which side chain would most likely be found in the interior of a protein, away
from the aqueous environment?
a.
-CH2OH
b. -CH2SH
с. -СH-CООН
d. -CH2CONH2
e.
4) What would happen if you treated Alanine (above) with an aqueous solution of:
a. HCI
HO
b. NaOH
HO
NH₂
NH₂
HCI
NaOH
D. Consider the amino acids glycine, proline and lysine.
a. How many tripeptides can be formed from these three amino acids if each is used
only once in the structure?
b. Using three-letter abbreviations for the amino acids, give the sequence of each of
the possible tripeptides.
c. Draw the structure of the dipeptide that has proline at its N-terminal amino acid
and glycine as its C-terminal amino acid. Circle each peptide bond.
Chapter 17 Solutions
Essential Organic Chemistry (3rd Edition)
Ch. 17.1 - a. Explain why, when the imidazole ring of...Ch. 17.2 - Prob. 2PCh. 17.3 - Prob. 3PCh. 17.3 - Prob. 4PCh. 17.3 - Prob. 6PCh. 17.4 - Calculate the pI of each of the following amino...Ch. 17.4 - a. Which amino acid has the lowest pI value? b....Ch. 17.5 - What aldehyde is formed when valine is treated...Ch. 17.5 - Prob. 10PCh. 17.5 - Prob. 11P
Ch. 17.5 - Prob. 12PCh. 17.6 - Prob. 13PCh. 17.6 - What amino acid would be formed using the...Ch. 17.6 - What amino acid would be formed when the aldehyde...Ch. 17.7 - Pig liver esterase is an enzyme that catalyzes the...Ch. 17.8 - Prob. 17PCh. 17.8 - Prob. 18PCh. 17.8 - Prob. 19PCh. 17.8 - Prob. 20PCh. 17.10 - Prob. 21PCh. 17.10 - Prob. 22PCh. 17.10 - Why does cyanogen bromide not cleave on the C-side...Ch. 17.10 - Prob. 24PCh. 17.10 - Prob. 26PCh. 17.12 - Prob. 27PCh. 17.13 - a. Which would have the greatest percentage of...Ch. 17 - Draw the predominant form of the following amino...Ch. 17 - What is the pI of serine?Ch. 17 - Prob. 31PCh. 17 - Prob. 32PCh. 17 - Which would have a higher percentage of negative...Ch. 17 - Draw the form of aspartate that predominates at...Ch. 17 - Prob. 35PCh. 17 - A professor was preparing a manuscript for...Ch. 17 - a. Why is the pKa of the glutamate side chain...Ch. 17 - Prob. 38PCh. 17 - Determine the amino acid sequence of a polypeptide...Ch. 17 - Prob. 40PCh. 17 - Prob. 41PCh. 17 - Three peptides were obtained from a trypsin...Ch. 17 - Prob. 43PCh. 17 - After the polypeptide shown here was treated with...Ch. 17 - The disulfide bridges of a polypeptide were...Ch. 17 - -Amino acids can be prepared by treating an...Ch. 17 - Reaction of a polypeptide with carboxypeptidase A...Ch. 17 - Prob. 48PCh. 17 - Prob. 49PCh. 17 - Show how valine can be prepared by a. a Strecker...Ch. 17 - Prob. 51PCh. 17 - Why is proline never found in an -helix?Ch. 17 - Determine the amino acid sequence of a polypeptide...Ch. 17 - Prob. 55PCh. 17 - A chemist wanted to test his hypothesis that the...Ch. 17 - A normal polypeptide and a mutant of the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain why, when the guanidino group of arginine is protonated, the double-bonded nitrogen is the nitrogen that accepts the proton.arrow_forward1. True or false a) Kappa- and Iota- carrageenans contain 3,6-anhydro-a-D-galactopyranosyl residues. b) In kappa and iota carrageenans, gels are formed through double helical formation of two polysaccharide segments via covalent interactions.arrow_forwardWhy are Guanidins and Amidins such strong bases?arrow_forward
- Why is the Alanine predominantly zwitterionic at its PI?arrow_forwardA. Draw the structure of L-valine in astrongly basic solution. B. What is the charge of this amino acid in a strongly basic solution?arrow_forwardExplain why, when the guanidino group of arginine is protonated, the double-bonded nitrogen is thenitrogen that accepts the proton. 2 H+ + NH2NHCH2CH2CH2CHNH +NH3CO−O CO−O H2NC NHCH2CH2CH2CHarrow_forward
- 1. Determine the isolation points (kasein) of alanine and argininearrow_forward1. structure of L-valine in a strongly basic solution. 2. What is the charge of this amino acid in a strongly basic solution? 3. What is the pH of D at its isoelectric point? 4. show the structure of this amino acid at its isoelectric point. 5. What is the charge of this amino acid in a strongly acidic solution?arrow_forwardA. Draw the structure of L-valine in a strongly basic solution. B. What is the charge of this amino acid in a strongly basic solution? C. What is the pH of D at its isoelectric point? D. Show the structure of this amino acid at its isoelectric point. E. What is the charge of this amino acid in a strongly acidic solution?arrow_forward
- 1. At what pH is the side chain of arginine 65% ionized? 2. At what pH is glutamate 25% ionized? 3. At what pH is the side chain of histidine 4/5 ionized? 4. At what pH is the side chain of cysteine 15% ionized? 5. At what pH is tyrosine 5/8 ionized?arrow_forwardWhich one is Second prominent form of arginine at pH 6.0 in the picture below?arrow_forwardThe RGD peptide is critical for the establishment of the extracellular matrix of animal cells. a. Draw the structure of the RGD peptide at physiological pH (pH = 7). b. What is the charge of this peptide at physiological pH? At high pH (pH> 13)? Low pH (pH < 3)?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY